FOR IMMEDIATE RELEASE
April 28, 2021
New York, NY
Contact: Joanne Watters – jwatters@informationmediary.com
IMC sets new clinical research standard with CertiScan® 2.0 end-to-end medication adherence analytics, disrupting smart device marketplace with customer-friendly ROI-driven data plans
Information Mediary Corp. (IMC), global leader in medication adherence solutions since 2002, is announcing CertiScan® 2.0 (www.certiscan.net), upgrading the industry by setting a new GxP standard for clinical researchers. CertiScan® 2.0 improves transparency and data capture in medication adherence management for all stakeholders involved in running clinical trials. CertiScan® 2.0 will vastly improve ROI on research through its uniquely integrated approach for all stakeholders conducting clinical research trials and specialty pharma development.
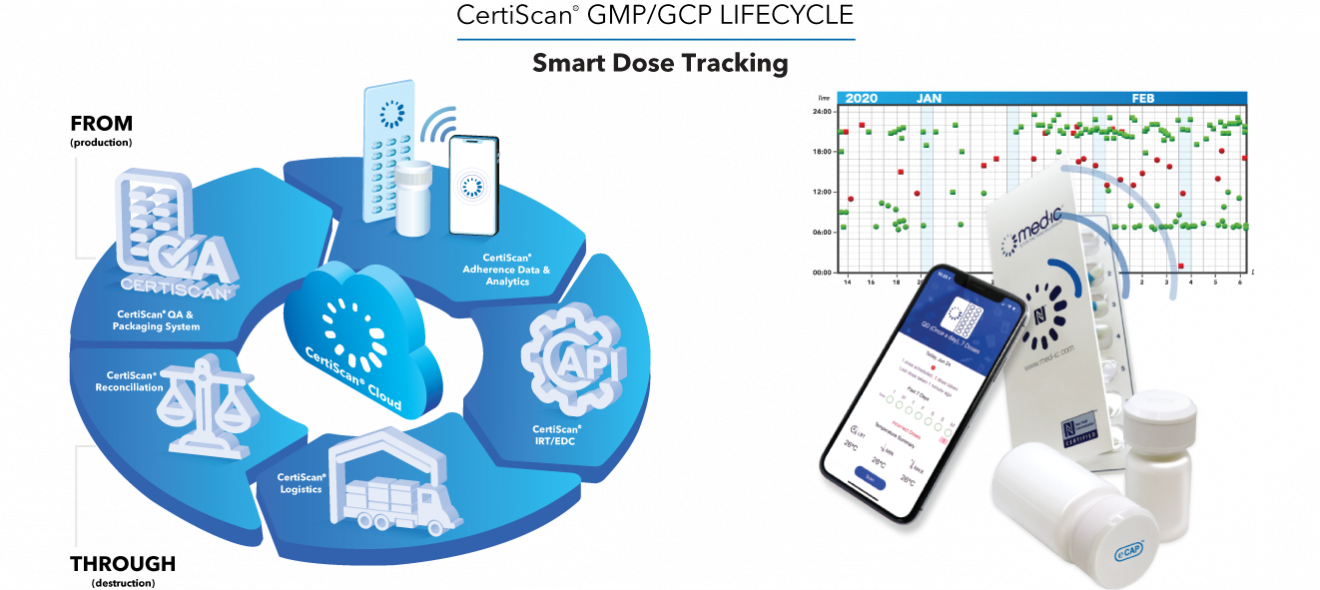
CertiScan® 2.0 is a vertically integrated SaaS, where GMP (devices) meet GCP (data and analytics). CertiScan® 2.0 provides dose-level traceability, quality assurance and content monitoring (including patient usage and storage temperature) of drug supply from packaging to dispensation and destruction. By combining it with patient dose-taking adherence data makes it a proven and insightful tool for rapid successful drug research.
“The ultimate goal is to make Clinical research faster and less expensive. The ROI on our approach is tremendous; we call it the $100 Million opportunity for clinical trials” says Joanne Watters, Director of Global Customer Support at the recent Global Clinical Supplies Conference. “CertiScan® 2.0 offers a comprehensive GxP cloud portal facilitating dose-level lifecycle data, analytics, IRT reconciliation and logistics; all connected to our best-in-class adherence devices to monitor, analyze and improve patient adherence with the prescribed medication regimen.”
CertiScan® 2.0 Cloud easily integrates with partner IRT and EDC systems, and is compliant with HIPAA, GDPR, 21 CFR Part 11, and EU Annex 11. Based on data generated, analytics and machine learning algorithms, CertiScan® 2.0 can prompt, coach, remind or otherwise encourage diverse patient populations to adapt their adherence behavior toward the prescribed drug regimen.
INTEGRATION
IMC is also the creative provider of Med-ic®, the only Smart Dose Blister ever submitted for FDA priority review resulting in a blockbuster drug approval. This amazing ecosystem fully integrates with IMC’s other award-winning NFC-Forum certified devices:
- Med-ic® Smart Blister and Vial Package
- eCAP Smart Pill Bottles, rated the most accurate devices in the industry
- CoolBlue-AI Smart Syringe Unit Package
- eBOXTM Smart MultiMed Box
- Med-ic® Polypharmacy Card
- CoolBlue®-AI Intelligent Temperature Monitoring
Information Mediary’s Chief Technology Officer Dean Brotzel, and his team have brought over 15 years of experience, involving tens of thousands of patients and over 20 Million smart package dose data points, to drive development of the CertiScan®2.0 Adherence Solution. Deploying this new solution removes all integration difficulties, barriers to adoption, vendor issues and quality concerns. “We seamlessly support our customers as well as various EDC, IRT and associated vendor platforms from medication packaging operations through to clinical research sites and onto final destruction”, explains Brotzel.
ADOPTION
In a significant step toward standardizing and simplifying medication adherence management in clinical research and specialty pharma, IMC now offers their GxP hardware, software and CertiScan® services as a subscription model, eliminating the final barrier to adapting smart dose analytics in clinical research: cost and supply management.
“Customers signing up with us don’t need to carry the full burden of high value smart devices but instead pay-for-use based on the data generated and/or patients enrolled on a monthly basis”, explains Dean Brotzel in advance to this week’s HITLAB “Disruptors and Disrupted” HealthTech conference held virtually from New York City on April 29, 2021.
Dr. Stan Kachnowski, Executive Director of HITLAB calls CertiScan® 2.0 “perhaps the biggest improvement to clinical research processes since barcodes became mandatory for labeling clinical supplies over 20 years ago; a truly disruptive solution that is sure to strengthen results from crucial clinical investigations”.
About Information Mediary Corp. (IMC)
IMC is the global leader in smart medication and adherence analytics solutions. IMC manufactures iOS and Android NFC-enabled Med-ic Smart Blister Packages, eCAP Smart Medication Bottles and the temperature aware Medic Coolblue Syringe Pack. IMC is active in the AI-enabled digital health field and offers its own HIPAA compliant, secure CertiScan® 2.0 adherence cloud. IMC was founded in 2002 and has supplied leading clinical research groups with smart adherence technologies since 2005. www.informationmediary.com